Mineral Build-Up
Most naturally hard water, due to the presence of carbon dioxide (CO2), will assume a temporary hardness (calcium bicarbonate). Through the process of heating water, dissolved solids (lime) precipitate out of the water and are deposited in the water heater tank.
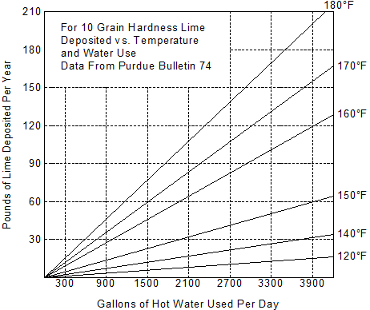
The chart below, developed from test data provided in Purdue University Bulletin No. 74, show the rate of precipitation of dissolved solids in pounds per year, at various water temperatures and usage.
The chart is based on 10 grain temporary hardness water. For other grain hardness, multiply the “Pounds Lime Deposited Per Year” data by the new grain hardness converted to a multiple of 10. For example, 20 grains hardness + 2 times the data; 15 grains = 1.5 times the data; 5 grains = 0.5 times the data; etc.
To use the chart:
- Determine the amount of hot water being used in a day.
- Know the temperature of the heated water.
- From the amount of water being used, project a vertical to the point of interception with the water temperature line. From this point perfect a horizontal line to the point of interception with the vertical line denoting Pounds Lime Deposited Per Year and read the result.
For example: Water usage is 3300 gallons per day; water temperature is 140° F. Projecting to points of interception as described above, we find 27 pounds of lime will be deposited in one year. With the same water usage per day but a change in temperature to 180° F, we find 168 pounds of lime will be deposited in one year – this is approximately six times as much lime as deposited by water heated to 140° F….. Following the simple steps above, lime accumulation per year for any grain hardness can be forecast.

 USA
USA CAN
CAN

